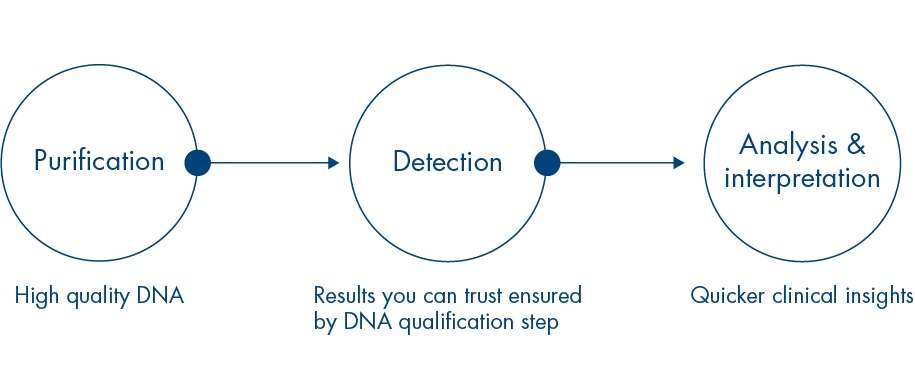
Now the therascreen EGFR test covers even more clinically validated mutations to deliver more of the valuable information you need to make the best therapeutic decisions.
With the recent approval of GILOTRIF to treat patients with additional EGFR mutations, our therascreen EGFR test now covers even more actionable mutations, and helps treat an even broader group of NSCLC patients.